Margaret (Peggy) C. Etter Lecture Videos, 1992
These two videos are shared in memory and recognition of the contributions of Prof. Peggy Etter to solid-state organic chemistry, in the hope of broadening the opportunity for young scientists to be influenced by her science and her message.
For some background on Prof. Etter, see:
"A Personal Recollection," by Joel Bernstein (Cryst. Growth Des. 2016, 16, 3, 1135–1143): pubs.acs.org/doi/10.1021/acs.cgd.5b01296;
the Chemistry of Materials special issue: pubs.acs.org/toc/cmatex/6/8;
the Margaret C. Etter Memorial Lecture in Materials Chemistry: https://cse.umn.edu/chem/margaret-c-etter-memorial-lecture-materials-chemistry/; and
the American Crystallographic Association Margaret C. Etter Early Career Award : www.amercrystalassn.org/etter-early-career.
I thank the late Prof. Joel Bernstein (see Cryst. Growth Des. 2020 special issue: pubs.acs.org/doi/10.1021/acs.cgd.0c00340) who, I believe, had the original VHS recordings transcribed to digital format and, to my delight, was kind enough to share them with me (and, I'm sure, many others) during the 35th crystallography course, "Diversity Amidst Similarity", in Erice, Italy in the summer of 2004. I thank Prof. Mike Ward (NYU, Chemistry) for reaching out to the Etter family and the University of Minnesota, Department of Chemistry and garnering their blessing to publish these videos in this open-access environment. ENJOY!!! - Travis Holman
A Potpourri of Crystal Chemistry, Part I: Phase Transformations and Crystal Reactions Department of Chemistry, University of Minnesota, January 1992
www.youtube.com/watch?v=wYpXXjPjxwE&t=14s
A Potpourri of Crystal Chemistry, Part II: Crystal Growth and Morphology Department of Chemistry, University of Minnesota, January 1992
www.youtube.com/watch?v=p6eFlkzuums&t=2296s
A third video in this series was recently shared by the Department of Chemistry at the University of Minnesota:
Margaret C. Etter Lectures Part III: Graph Sets - January, 1992
www.youtube.com/watch?v=PQMNaE43YUo
For some background on Prof. Etter, see:
"A Personal Recollection," by Joel Bernstein (Cryst. Growth Des. 2016, 16, 3, 1135–1143): pubs.acs.org/doi/10.1021/acs.cgd.5b01296;
the Chemistry of Materials special issue: pubs.acs.org/toc/cmatex/6/8;
the Margaret C. Etter Memorial Lecture in Materials Chemistry: https://cse.umn.edu/chem/margaret-c-etter-memorial-lecture-materials-chemistry/; and
the American Crystallographic Association Margaret C. Etter Early Career Award : www.amercrystalassn.org/etter-early-career.
I thank the late Prof. Joel Bernstein (see Cryst. Growth Des. 2020 special issue: pubs.acs.org/doi/10.1021/acs.cgd.0c00340) who, I believe, had the original VHS recordings transcribed to digital format and, to my delight, was kind enough to share them with me (and, I'm sure, many others) during the 35th crystallography course, "Diversity Amidst Similarity", in Erice, Italy in the summer of 2004. I thank Prof. Mike Ward (NYU, Chemistry) for reaching out to the Etter family and the University of Minnesota, Department of Chemistry and garnering their blessing to publish these videos in this open-access environment. ENJOY!!! - Travis Holman
A Potpourri of Crystal Chemistry, Part I: Phase Transformations and Crystal Reactions Department of Chemistry, University of Minnesota, January 1992
www.youtube.com/watch?v=wYpXXjPjxwE&t=14s
A Potpourri of Crystal Chemistry, Part II: Crystal Growth and Morphology Department of Chemistry, University of Minnesota, January 1992
www.youtube.com/watch?v=p6eFlkzuums&t=2296s
A third video in this series was recently shared by the Department of Chemistry at the University of Minnesota:
Margaret C. Etter Lectures Part III: Graph Sets - January, 1992
www.youtube.com/watch?v=PQMNaE43YUo
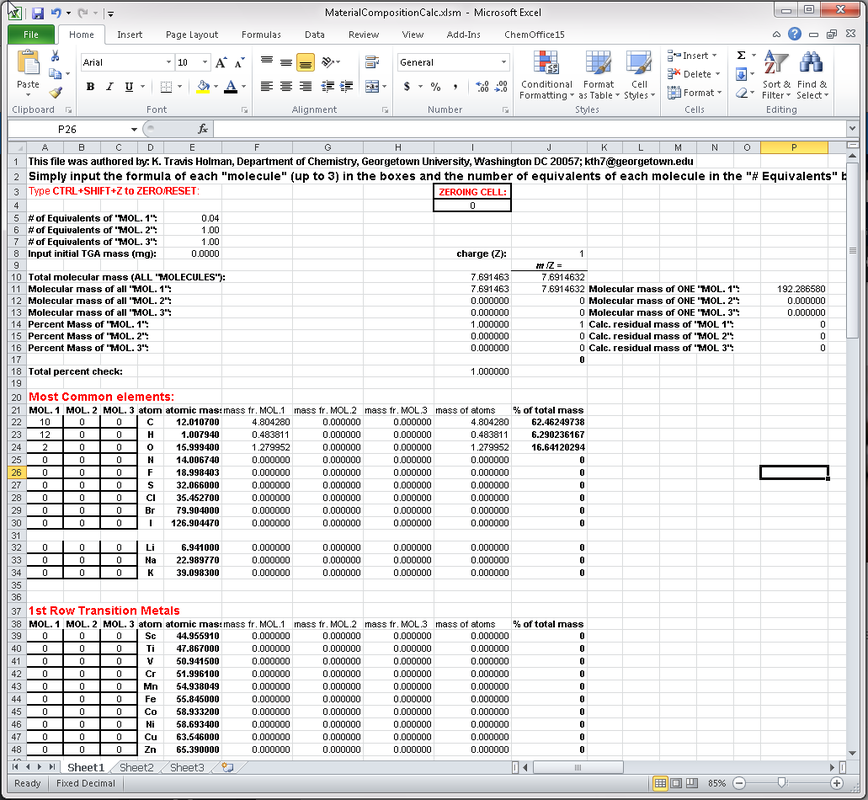
Material Composition Calculator for Inclusion Compounds
The Materials Composition Calculator is a free Microsoft Excel spread sheet application developed by Travis Holman to assist in quick calculation of average molecular weights, elemental percent compositions, theoretical crystal density, and percent mass composition of each component of an up to three-component solid. Conveniently, the material may be stoichiometric or non-stoichiometric, as per the User's need, and up to ternary composition. Simply input the formula of each component (MOL. 1-3), choose the stoichiometries of each component (# of Equivalents of MOL. 1-3), and the application will calculate the percent mass composition of each element in the material and each component. If the crystal unit cell and Z are known, the density can also be calculated. The application is particularly suited for rapidly determining, by trial and error, the relative stoichiometry of each component in comparison to thermal gravimetric analysis (TGA) data (% mass loss). It is also useful for calculation of expected combustion (elemental) analysis data for multicomponent, even non-stoichiometric materials. It's great for salts, solvates/hydrates, cocrystals, and other inclusion compounds, and doesn't require fixed stoichiometry.
Example: A ternary partial solvate of composition: 2 equivalents of phenyl succinic acid (PSA, C10H10O4), 1.5 equivalents of pyridine (pyr, C5H5N), and 0.16 equivalents of ethanol (EtOH, C2H6O). consists of 64.96% carbon 5.58% hydrogen 25.38% oxygen, and 4.08% nitrogen and the percent masses of each component are as follows: PSA, 75.5% pyr, 23.1%, EtOH, 1.4% Or, equivalently, TGA analysis of an ill-defined, partial ethanol solvate of a 4:3 PSA:pyr cocrystal shows a 1.4% mass loss just above room temperature, followed by a 23.1% mass loss above 100 degrees Celsius. The bulk material therefore contains ~0.16 equivalents of ethanol. Data entry for this calculation takes about 30 seconds, and the stoichiometries can be easily tweaked to match TGA or combustions/elemental analysis data. If you find yourself using it, please shoot us an e-mail and/or acknowledge it in any publications. |
| ||||||